

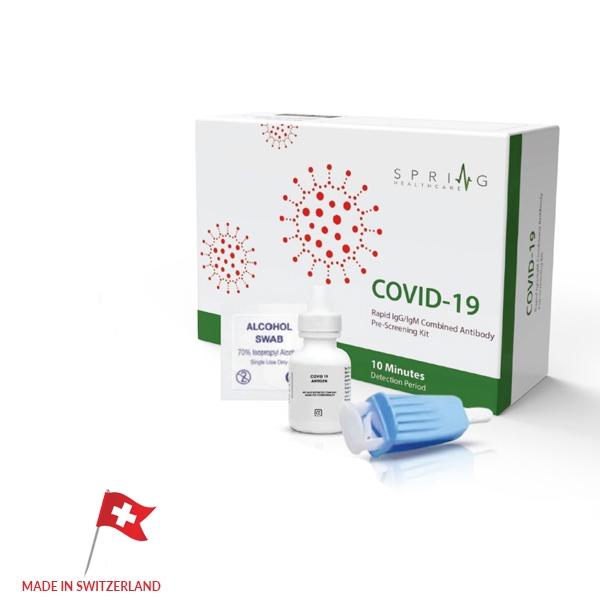
COVID-19 Rapid Test

Made in Switzerland, the FDA approved CoronaVirus Rapid Test can check a patient for the Coronavirus antibodies in 10 minutes. By checking for two Corona antibodies through a patient’s self-taken blood sample, the test can identify current and previous infections in patients, as well as levels of immunity. While most accurate Corona tests require laboratory equipment which can delay results for several hours to days, the Corona Virus Rapid Test needs no additional materials to be completed. While there have been other tests in China as well as other countries that have surfaced with similar rapid tests methods, none have proven to be accurate enough for screening public patients and providing proper diagnosis.
The FDA approved CoronaVirus Rapid Test from Switzerland has shown to have over 98% accuracy results, with several controls and sample tests having been performed prior to its recent release. With the all new, FDA and SGS approved CoronaVirus Rapid Test, screening patients will no longer involve long waits, large costs, and inaccurate results. Our test allows professionals to quickly screen any patients with symptoms while allowing the opportunity for patients to test themselves from the comfort of their home without having to come in contact with other individuals and risk further spread. At US Ophthalmic, we are putting all our resources to fight this global pandemic; we know that this test will help us overcome this challenge by keeping patients properly diagnosed and safe.
HOW TO WORKS
CURRENT METHOD
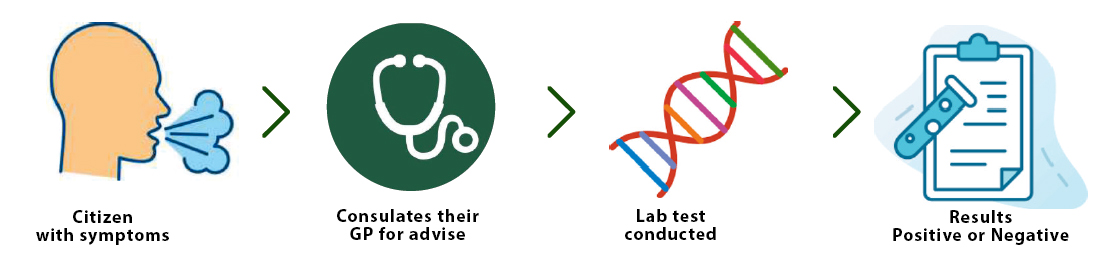
HOW TO WORKS
PRE-SCREENING
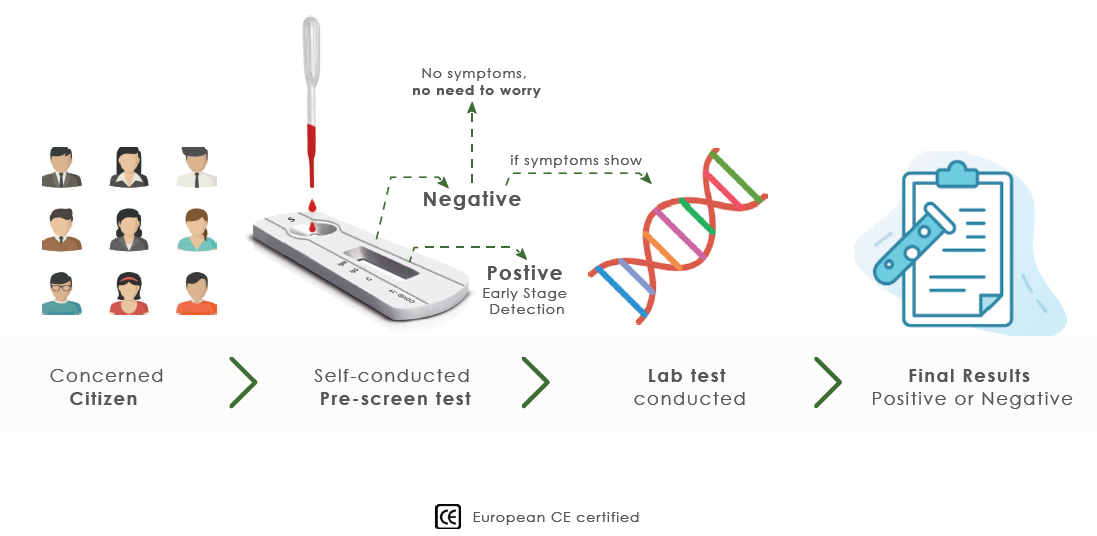

HOW TO WORKS
BENEFITS of pre-screening
• Exponentially faster
• No Queues, No Wait time for results
• Exponentially more cost e ective
• Early Detection & Elimination
• Effort less, and accessible to everyone nationwide, instantly
• Viable precautionary step
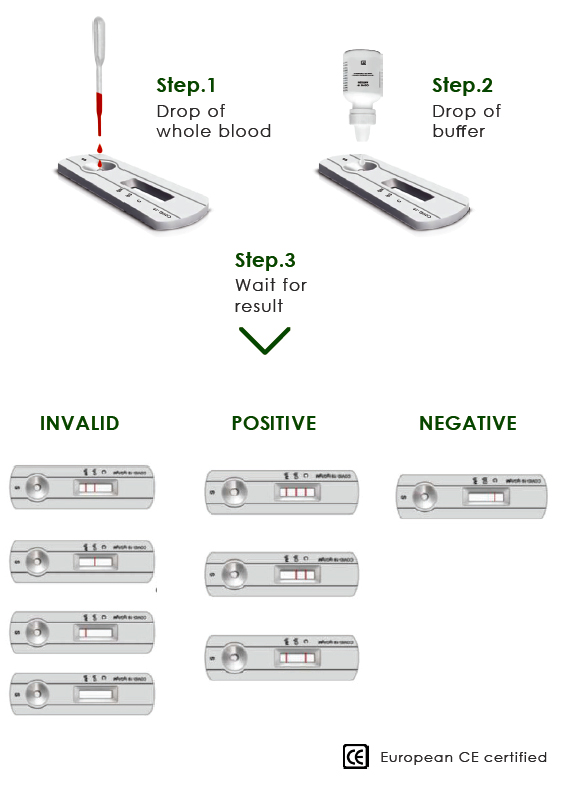
HOW TO WORKS
Detection Period 10 MINUTES
• Easy operation without requirement of any Doctor or Professional Nurse
• No special equipment storage and transportation conditions required
• Works with whole blood, serum, and plasma
• Tests for 2 antibodies IgM and IgG simultaneously
• Instant Field screening98%+ACCURACY
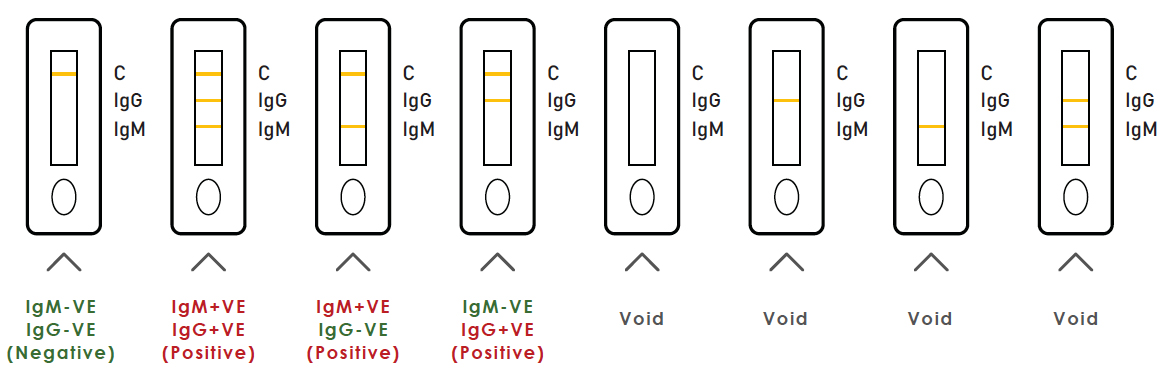
| Results | Interpretation |
| IgM+VE, IgG+VE | Suspected recent infection of 2019-nCov |
| IgM+VE, IgG-VE | Suspected recent infection of 2019-nCov |
| IgM-VE, IgG+VE | Patient Suspected to have past infection |
| IgM-VE, IgG-VE | Antibody for COVD-19 Virus undetected OR loW IgG/IgM level belo w limit of detection |
TECHNICAL REVIEW
| Fluorescence PCR | CLIA | Colloidal Gold Method (our method) | |
| Detection Susbtance | Nucleic acid | Antibody | Antibody |
| Type of Sample | Nasopharyngeal swabs, sputum, alveolar lavage fluid | Serum / Plasma | Serum/ Plasma / Whole blood |
| Time to get Result | 2 hrs | 20 min | Fastest 10 minutes |
| Instrument Needed or Not | Yes | Yes i 3000, i 1000 |
Not needed |
| Laboratory Requirement | High | Relatively High | Average |
| Product Usage | Confirming diagnosis | Nucleic acid negative sample reviewing or high-volume sample detection | Nucleic acid negative sample reviewing or basic hospital sample testing |
| Colloidal Gold Method: The 2019-nCOV IgG/IgM Rapid Test Device using this Method is a rapid chromatographic immunoassay for the qualitative detection of IgG & IgM antibody of Coronavirus in human whole blood, serum, or plasma | |||
RT-PCR Vs SARS-CoV-2 IgM Ab Rapid Test
Comparative Test Summary
In this trial, 1300 clinical samples were selected. There were 300 positive samples and 1000 negative samples.
The SARS-CoV-2 IgM Ab rapid test and the SARS-CoV-2 RT-PCR test were detected simultaneously and the Sensitivity (positive coincidence rate), Specificity (negative coincidence rate), and Accuracy (total coincidence rate) were calculated:
| Antibody | Sensitivity | Specifity | Accuracy |
| IgG | 93% | 97.5% | 96.5% |
| IGM | 92% | 96% | 95.8% |
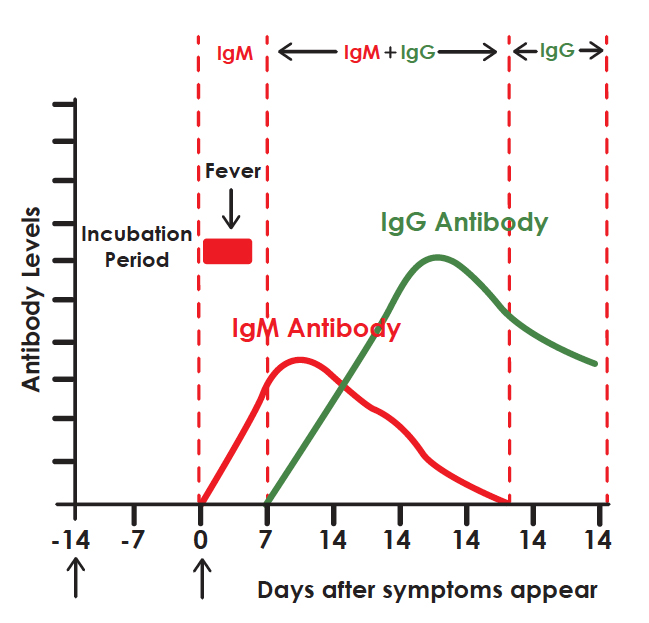
DIAGNOSTIC PROCESS
It is widely accepted that IgM provides the first line of defence during viral infections, followed by the generation of adaptive, high a nity IgG responses for long term immunity and immunological memory. Therefore testing of COVID-19 IgM and IgG antibodies is an ef ective method for the rapid diagnosis of COVID-19 infection. Furthermore, detection of COVID-19 IgM antibodies tends to indicate a recent exposure to COVID-19, whereas detection of COVID-19 IgG antibodies indicates a later stage of infection. Thus, this combined antibody test could also provide information on the stage of infection.
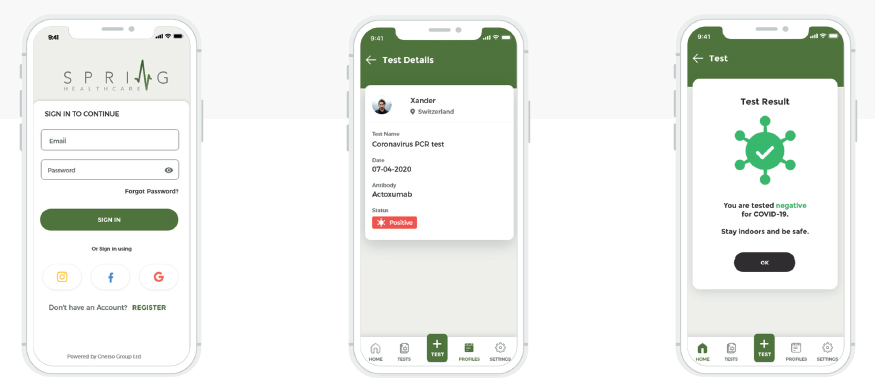
SPRING HEALTH DIAGNOSTIC APPLICATION
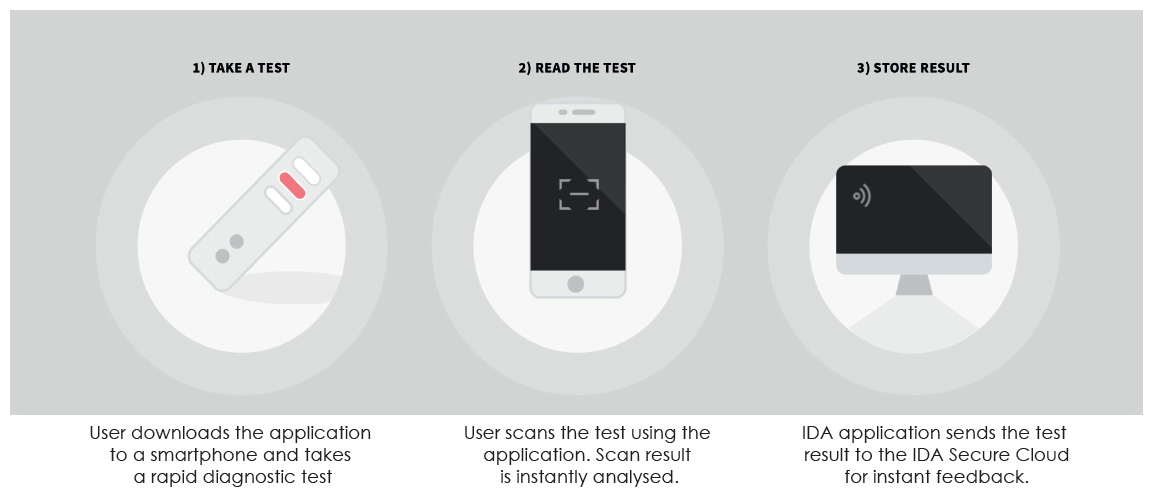
Spring Health has developed a disruptive mobile solution to capture diagnostic immediately and for analytics. The globally scalable end-to end solution has proven to be a powerful product and service.Spring Health Infectious Diseases Reader is a solution for healthcare professionals in Point of Care diagnostics for COVID-19. The Spring Health solution consists of a smartphone mobile reader application and a backend solution.The smart phone mobile reader application is used to digitize and analyse lateral flow tests, while the backend solution provides analysis, support and dynamic configuration for the supported lateral flow tests. With very minimal instructions even to untrained staff, the solution can be deployed to capture digitized diagnostic data. The data can be verified and analysed in 3 simple steps:
BENEFITS
* Immediate diagnostics without costly hardware setups – only a smartphone is necessary
* Web applications in the Secure Cloud ensure real-time medical guidance back to the mobile application user
* No attachments – no additional hardware – no additional trainings or wires are required
* Seamless data management integration into client / stakeholder information systems
* Disease surveillance and real time epidemic alerts via interactive mapping
* Lateral flow test quality control and quality assurance






Reviews
There are no reviews yet.